A Bucket Full Of Hot Water Cools From 80 To 75
Newtons Law of Cooling is useful for studying water heating because it can tell us how fast the hot water in pipes cools off. Q 115 g 100 C 4184 J g 1 C 1 48116 J Note the inclusion of the melted 15 g of ice.

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then
This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample.

A bucket full of hot water cools from 80 to 75. Please see the Owners Guide for you Storage Water. Specific heat capacity of water 4200 J kg-1K1 Calculate i the energy lost by the water as it cools to 0 C ΔQ mcΔθ energy lost by water 020 4200 20 1 mark. A bucket full of hot water is kept in a room and it cools from 75C to 70C in t1 minutes from 70C to 65C in t2 minutes and from 65C to 60C in t3 minutes.
The external water tray is to be used for draining the unit when the internal water tray is full. 160 86 85 833 82 80 78 75 150 785 76 75 73 70 67 685 140 71 69 67 64 60 555 50 130 65 615 58 545 50 44 375 120 57 54 50 45 40 33 25 110 50 46 415 36 30 21 12 100 43 38 33 27 20 11 -Example. A practical application is that it can tell us how fast a water heater cools down if you turn off the breaker when you go on vacation.
A bucket full of hot water is kept in a room and it cools from 75C to 70C in T1 minutes from 70C to 65C in asked Jun 19 2019 in Physics by Helisha 688k points heat. Position the external water tray under the drain at the bottom rear of the unit then remove the cap and plug and let water fill the tray. And youre getting a little hot and bothered literally.
Let us determine how much ice will be melted by the 160038 J. In fact it seems your AC has trouble hitting any temperature below 80 degrees. Ad Find China Manufacturers Of Solar Water Heaters.
Q3 A tray containing 020 kg of water at 20 C is placed in a freezer. Read on to learn how to apply the heat capacity formula correctly to obtain a valid result. This quantity should be equal to approximately 150 of the hot water used as water expands by this volume when heated.
Only input whole numbers do not use a comma or point. To heat the same water volume in half the time 30 minutes would need twice the heating power ie 7kW. So you set your AC thermostat below 80 degrees but noticed that your air conditioners having trouble reaching your set temperature.
The calculators on this page compute how long it takes to heat water how much energy is consumed and how much heating power is required. Click hereto get an answer to your question A bucket full of hot water is kept in a room and it cools from 75C to 70 in T1 minutes from 70C to 65C in. Then a t1 t2 t3 b t1 t2 t3 c t1 t2 t3 d t1 t2 t3.
B t1 t2 t3. The calculators assume 100 efficiency and no loss of energy during the heating process. Water Heating Calculator for Time Energy and Power.
160038 J x 18015 gmole 6020 Jmole x 479 g --- the answer to b 4 Since ice remains in contact with the liquid water the final temperature of the mixture will be zero degrees Celsius. A The temperature of the water drops to 0 C in 10 minutes. However if it discharges more than a bucket full of water in 24 hours there may be another problem.
2 Determine heat need to raise 115 g of water from 0 to 100 C. A bucket full of hot water is kept in a room and it cools from 75C to 70C in T1 minutes from 70C to 65C in asked Jun 19 2019 in Physics by Helisha 688k points heat. Do this until no more water comes out of drain and when completely drained reinstall the plug and drain cap.
Ad Find China Manufacturers Of Solar Water Heaters. Note that the 100 g of water is not mentioned yet. 1 Desired mixed outlet water temperature 140F 71 of hot water 180F 2 Hot water supply stored water temp 180F 29 of cold water.
Converesely if we only use half the heating power 175kW it will take twice as long to heat up to desired temperature ie 2 hours. A bucket full of hot water is kept in a room and it cools from 75C to 70C in t1 minutes from 70C to 65C in t2 minutes and from 65C to 60C in t3 minutes. A swimming pool at 30C is at a lower temperature than a cup of tea at 80C.
If we only have a 1kW element available we will expect a heat up time in excess of 3 hours. We know this from the presence of the ice. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 K.
3 The warm water does not provide sufficient energy to melt all the ice. Click hereto get an answer to your question A bucket full of hot water cools from 75C to 70C in time T1 from 70C to 65C in time T2 and from 65C to 60C in time T3. But the swimming pool contains more water so it stores more internal energy than the cup of tea.
Also notice that the water was at zero C.

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes Youtube
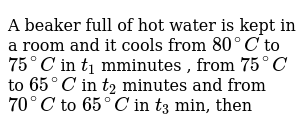
A Beaker Full Of Hot Water Is Kept In A Room And It Cools From 80

A Vessel Full Of Hot Water Is Kept In A Room And It Cools From 80

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes Youtube
A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes From 70 C To 65 C In T 2 Minutes And From 65 C To
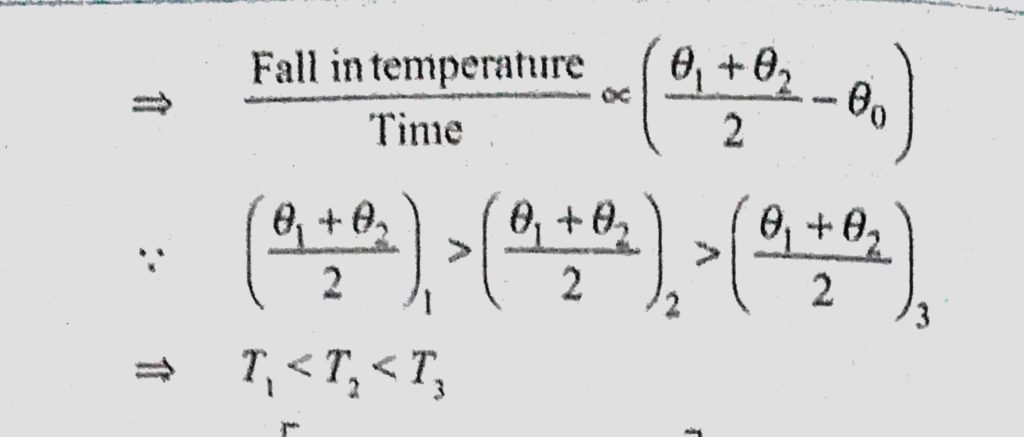
A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes From 70 C To 65 C In T 2 Minutes And From 65 C To

Post a Comment for "A Bucket Full Of Hot Water Cools From 80 To 75"