A Bucket Full Of Hot Water Cools From 75 To 70
A 500 g sample of aluminum specific heat capacity 089 J g 1 C 1 and a 1000 g sample of iron specific heat capacity 045 J g 1 C 1 are heated to 1000 CThe mixture of hot iron and aluminum is then dropped into 919 g of water at 237 C. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample.

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes From 70 C To 65 C In T 2 Minutes And From 65 C To
Hiker B has a body mass of 70 kg and carries a 20-kg pack.

A bucket full of hot water cools from 75 to 70. Heat the water in the tub without adding more water. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 K. Click hereto get an answer to your question A bucket full of hot water cools from 75C to 70C in time T1 from 70C to 65C in time T2 and from 65C to 60C in time T3.
What is the specific heat of the metal. The velocity with which thermal radiation. A bucket full of hot water is kept in a room and it cools from 75C to 70C in T1 minutes from 70C to 65C in asked Jun 19 2019 in Physics by Helisha 688k points heat.
A The temperature of the water drops to 0 C in 10 minutes. Convection radiation and diffusion. The time taken by water and liquid to cool from 70oC to 60oC is 3 min and 95 sec respectively.
Ad Find China Manufacturers Of Solar Water Heaters. A bucket full of hot water is kept in a room and it cools from 75C to 70C in t1 minutes from 70C to 65C in t2 minutes and from 65C to 60C in t3 minutes. No way to tell.
Only input whole numbers do not use a comma or point. The calculators on this page compute how long it takes to heat water how much energy is consumed and how much heating power is required. Heat can be transported in three ways.
The specific heat of the liquid will be. Then NCERT 80 CBSE 95 MHT-CET 99 a t 1 t 2 t 3 b t 1 t 2 t 3 c t 1 t 2 t 3 d t 1 t 2 t 3. Transfer the water to a smaller container.
Add hot water to the tub. All the ice melted and the temperature in the container rose to 100 C. Water Heating Calculator for Time Energy and Power.
The calculators assume 100 efficiency and no loss of energy during the heating process. A bathtub is half full of warm water. B t1 t2 t3.
Even if there is truly no resistance to heat flow diffusion from the water into the air the air around it still needs to transport. Click hereto get an answer to your question A bucket full of hot water is kept in a room and it cools from 75C to 70 in T1 minutes from 70C to 65C in. Q3 A tray containing 020 kg of water at 20 C is placed in a freezer.
Ad Find China Manufacturers Of Solar Water Heaters. A bucket full of hot water is kept in a room and it cools from 75C to 70C in t1 minutes from 70C to 65C in t2 minutes and from 65C to 60C in t3 minutes. Read on to learn how to apply the heat capacity formula correctly to obtain a valid result.
A bucket full of hot water cools from 75C to 70C in time T_1 from 70C to 65C in time T_2 and from 65C to 60C in. They are filled with 350 gm of water and 300 gm of a liquid equal volumes separately. Specific heat capacity of water 4200 J kg-1K1 Calculate i the energy lost by the water as it cools to 0 C ΔQ mcΔθ energy lost by water 020 4200 20 1 mark.
Then a t1 t2 t3 b t1 t2 t3 c t1 t2 t3 d t1 t2 t3. Add more water at the same temperature to the tub. Calculate the final temperature of the metal and water mixture assuming no heat loss to the surroundings.
1 Determine heat required to melt the ice. In Newtons experiment of cooling the water equivalent of two similar calorimeters is 10 gm each. A bucket full of hot water is kept in a room and it cools from 75C to 70C in t 1 minutes from 70C to 65C in t 2 minutes and from 65C to 60C in t 3 minutes.
A 350 g block of metal at 800 C is added to a mixture of 1000 g of water and 150 g of ice in an isolated container.
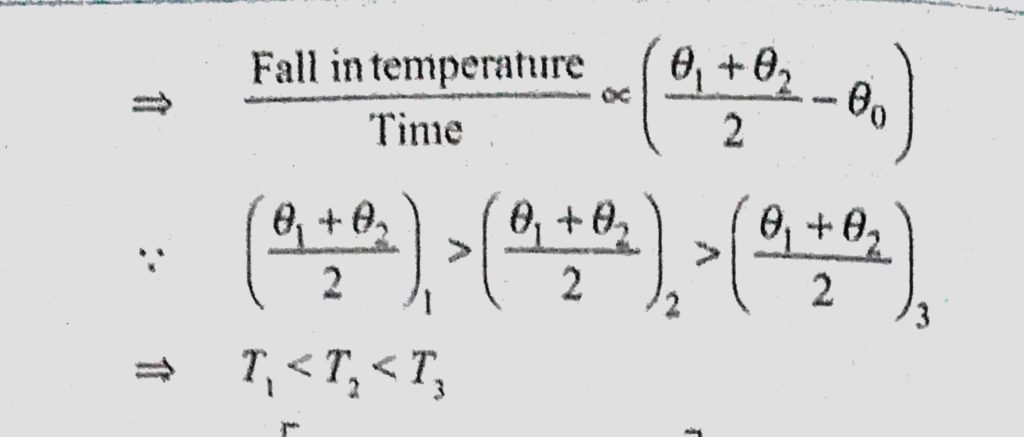
A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then
A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes Youtube

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T 1 From 70 C Youtube

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C In Time T3 Then

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes From 70 C To 65 C In T 2 Minutes And From 65 C To

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T1 From 70 C To 65 C In Time T2 And From 65 C To 60 C

A Bucket Full Of Hot Water Cools From 75 C To 70 C In Time T 1 From 70 C To 65 C In Time T 2 And From 65 C To 60 C In Time T 3 Then

A Bucket Full Of Hot Water Is Kept In A Room And It Cools From 75 C To 70 C In T1 Minutes From 70 C To 65 C In T 2 Minutes And From 65 C To

Post a Comment for "A Bucket Full Of Hot Water Cools From 75 To 70"